Catalyst Performance assessment is the important activity done by a Process Engineer in Chemical Process Plants. They are widely employed in Hydrogen Generation Plants, Syn Gas Production Units, Ammonia Production Facilities, Methanol Plants, Feed Gas Purification in Refineries etc. Therefore assessing the Catalyst activity and evaluating the life inevitable.
Need for Catalyst Performance Assesment
- First of all it is a great experience for Chemical Process Engineers to learn about Industrial Catalysis and related Chemical Process Calculations.
- It helps to optimise the Operating temperature, Pressure and Flowrates inside the Catalyst vessel to minimize the operating cost.
- It helps in benchmarking/ comparing the performance and Catalyst life with the industry best
- It is useful to predict the residual life of the catalyst which will help the plant engineers to place purchase order for the new catalyst when it is required.
Methods for Catalyst Performance Evaluation
There are many methods available for catalyst performance evaluation depending upon the type of application. Here are some of the most common methods used and details about how it is carried out.
Reactor Exit Composition
Analysis of reactor exit composition gives the good measurement of catalyst performance. When the reaction across the bed is not equilibrium limited, and the reaction conditions like Pressure, Temperature, Space Velocity, Inlet composition is constant, the reactor exit gases are analyzed and the catalyst performance can be evaluated. The example for this case is Eg: Hydrodesulfurisation in Refineries for feedstock purification. This reaction takes place in ZnO to Convert Hydrogen to Hydrogen sulphide. If the exit composition contains H2S it shows poor catalyst activity and the life is going to end and replenishment is needed.
Catalyst bed Temperature Profile
Exothermic reactions will show an increase in gas temperature across the bed. Normally a catalyst bed consists of vertically mounted thermocouple sheath in it to monitor the temperature across the bed. The temperature raise across the reactor gives good indication about catalyst life and catalyst activity.
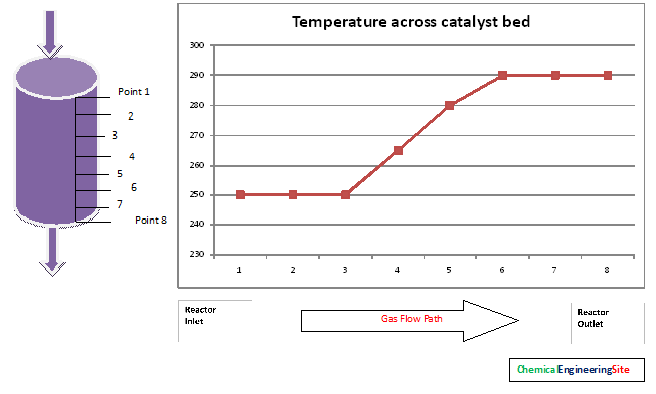
The first three points from the inlet thermocouple points shows no increase in temperature, this shows the catalyst activity is lost in those points. For a new catalyst this zone will not be there.
The next points ie from 3 to 6 activity persists and because of exothermic reaction the temperature raise is observed.
The points from 6 to 8 does not show raise in temperature. This shows there is no gas to react.
The active volume of the catalyst is the volume available for reaction to takes place. That is from point 3 to 8 in the above figure. The volume of catalyst can be calculated from the vessel dimensions and thermocouple locations.
Approach to Equilibrium (ATE)
Difference between the gas temperatures at the catalyst bed outlet to Equilibrium temperature corresponding to the gas composition at the exit of the bed is called as Approach to Equilibrium. This Approach to equilibrium method is only valid for equilibrium limited reactions like shift conversion in Hydrogen plants, Methanation reaction in Ammonia plant etc.
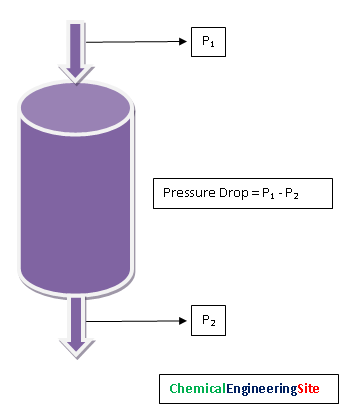
Pressure Drop
Pressure Drop across the vessel is the parameter that shows catalyst activity in real time. The Pressure drop across the vessel has to be measured at regular intervals. This gives significant information about catalyst life. Over a period of time this pressure drop will increase. If the pressure drop across the reactor bed is more the following may be because of the reasons like: Damage of Catalyst bed support, Carryover of dust/ catalyst particles from upstream vessels, Carbon formation, breakage of catalyst due to thermal cycling during startup/shutdown/upset conditions, Liquid carry over/ condensation in the pores etc
Enjoyed this Article Share it with Friends!
Related Books:
Buy Handbook of Industrial Catalysts By Lawrie Lloyd from Flipkart.com